|
|
 Originally Posted by BananaStand

Why doesn't the moon crash into the earth? For that matter, why doesn't the earth crash into the sun?
Isn't gravity supposed to pull things together?
OK. Everything (?) Ong said looks actually legit for Einstein's description of gravity
Einstein's General Relativity is the most accurate description of gravitation known so far.
I'll describe it in a Newtonian way. Newtonian gravity is less accurate, but a very good approximation for non-relativistic speeds.
I don't know your level of physics understanding, so I'll try to start with basic principles. Definitely ask questions if I get too technical. I love to break things down to a non-physicist's level of understanding. (I like to think Ong's excellent description of Einstein's gravity is at least a little bit due to this thread.) As I become better acquainted with your level of understanding, my answers will be more directly to the point you're asking.
I think, but I'm guessing, that the misunderstanding is between force, velocity and acceleration.
A re-phrasing of Newton's 2nd Law of motion:
F = ma
Force equals mass times acceleration. Bold letters indicate vectors. A vector has a magnitude and a direction.
Acceleration is defined as change in velocity over time, a = dv/dt.
Velocity is a vector. It has a magnitude (size, or length, if you like), called speed, and a direction.
Speed is not velocity. Velocity is speed in a direction. Speed is the magnitude of velocity.
Yes, outside of a physics conversation, these are inter-changeable, but in physics they're different.
OK. Forces cause accelerations. An acceleration is a change in velocity. Velocity is speed and direction.
On the face of it, any acceleration should be making the object speed up or slow down. It is true when the acceleration is parallel to the direction of motion that the object will speed up. It is true that when the acceleration is anti-parallel (pointing exactly opposite the direction of travel) the object will slow down. It's the direction bit that's messing with us.
Side track:
The intermediate value theorem
If f is a continuous function and f(x1) = y1, and f(x2) = y2,
then for value, y3, between y1 and y2,
there is at least one value, x3, which is between x1and x2
for which f(x3) = y3.

It basically says it's like this: If you're walking along some path, and at one point your elevation is X and at another point your elevation is Y, then you must have passed through all the in-between elevations at least once along your path, because you can't suddenly go from one height to another height w/o passing through the in-between heights.
Back on track:
So if there is some direction of acceleration which makes you slow down, and another direction of acceleration which makes you speed up, then there MUST be at least one direction of acceleration in between those 2 directions which will not change your speed, because directions are continuous.
This class of acceleration directions are those which are perpendicular to the object's direction of motion. These perpendicular accelerations cause the object to change its direction of motion (which is a change in velocity). So it is possible to accelerate w/o changing speed.
We're primed to talk about Newtonian orbits.
For any object moving in a circular path at a constant speed, the direction of acceleration is toward the center of the circular path. For an orbiting body, this acceleration is due to the gravitational interaction between the 2 bodies. Technically, the acceleration is toward the center of mass of the system, but with only 2 object, that center of mass is always between them, so the direction of acceleration is always pointed toward that in-between location. Since perspective is a matter of choice, it is always true that both bodies are orbiting around each other. Neither is stationary, because Newton's 3rd says that equal and opposite forces characterize all force interactions. Both bodies are pulled toward their center of mass, but neither falls into it, because they have some velocity which is perpendicular to the acceleration and keeps making them miss the exact center. Unless the size of the objects is such that they collide, they will perpetually orbit each other, 'cause no friction in space.
(Note that the "perpetually" part is subtly not true in Einstein's model.)
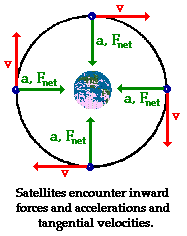
Technically, a satellite is any orbiting body. The moon is a satellite of Earth. Earth is a satellite of the sun, etc.
This still holds for elliptical orbits.
The orbiting body has some velocity which is perpendicular to the direction of acceleration. The direction of acceleration is still toward the center of mass of the system, but that center of mass is NOT always perpendicular to the object's path. Some of the acceleration is along or against it's path, either speeding it up or slowing it down, but some of the acceleration is making it turn toward the center of mass. Again, the object has some velocity which is toward or away from the center of mass of the system, but some which is perpendicular to it. If it doesn't collide with anything in its path, and some other details, it's in orbit.
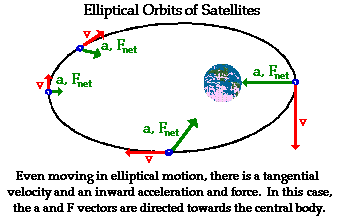
I discussed orbits in detail in this post.
|



 Reply With Quote
Reply With Quote





















